Why Test Pharmaceuticals?
Pharmaceuticals directly affect patient health. Even a minor lapse in quality or compliance can lead to regulatory action, market delays, costly recalls, and a loss of trust among patients and healthcare providers.
Regular testing ensures:
- Medicines are safe, effective, and consistent
- Compliance with GMP, GLP, ISO, ICH, and Pharmacopeia standards
- Faster market entry and export readiness
- Stronger protection of patients and brands

Types of Pharmaceutical Testing We Provide

Raw Material Testing
Why: To ensure APIs and excipients meet pharmacopeia standards.
What we test: Identity, purity, potency, microbial limits, contaminants.
Who needs it: API manufacturers, formulation units.

Finished Product Testing
Why: To confirm the final drug is safe, effective, and stable.
What we test: Assay, dissolution, disintegration, content uniformity, sterility, microbial limits.
Who needs it: Pharma manufacturers (tablets, injectables, syrups, etc.).

Stability Testing
Why: To establish shelf-life and storage conditions.
What we test: Accelerated and real-time stability studies, degradation profiling.
Who needs it: All pharma companies for regulatory filings.
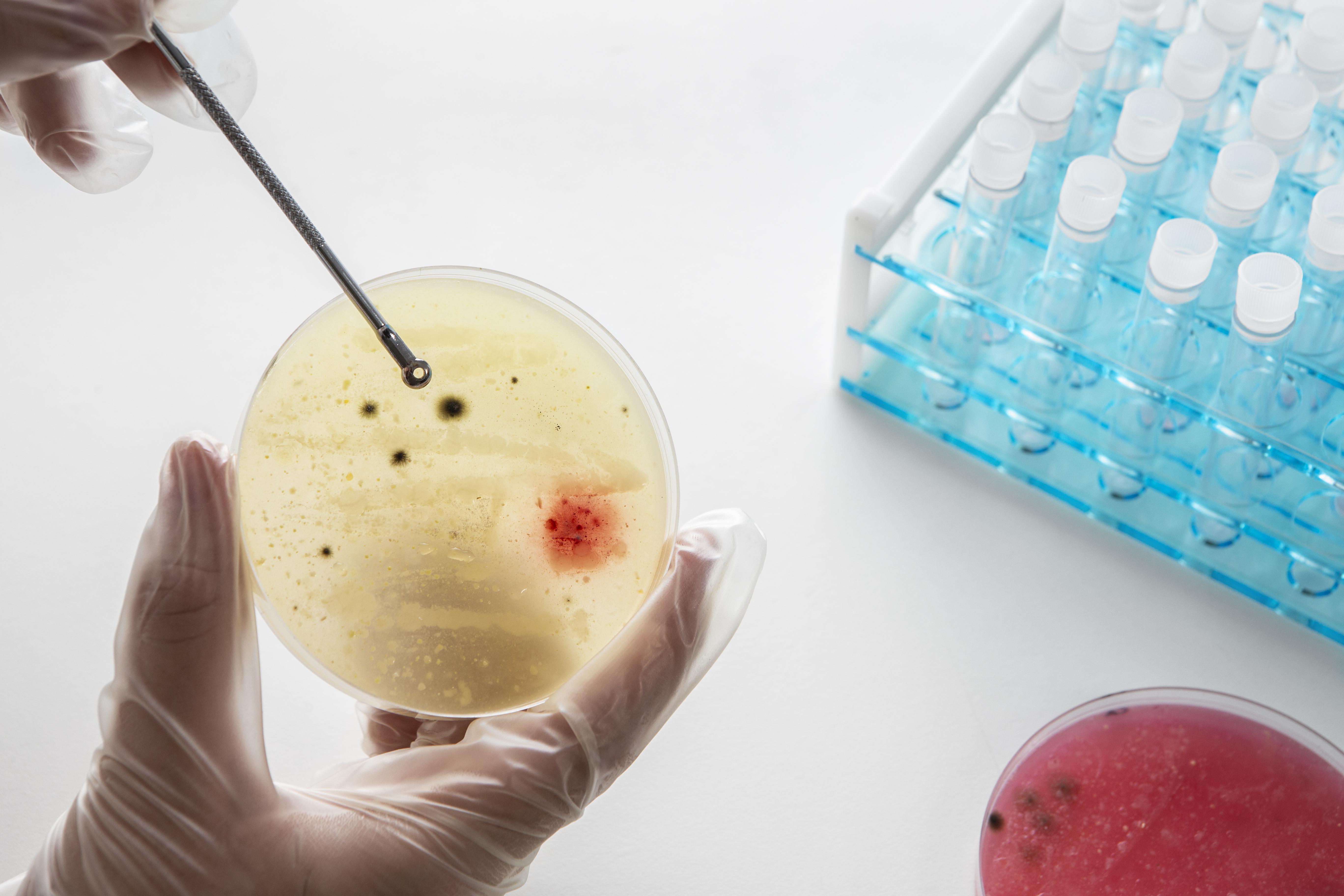
Microbial & Sterility Testing
Why: To prevent contamination risks in sterile and non-sterile products.
What we test: Sterility, endotoxins, bioburden, microbial limits.
Who needs it: Injectables, ophthalmic, and sterile dosage forms.

Utilities & Process Water Testing
Why: Water is a critical raw material in pharma and must meet pharmacopeia standards.
What we test: Purified water, WFI, clean steam, compressed gases.
Who needs it: All pharma manufacturing facilities.
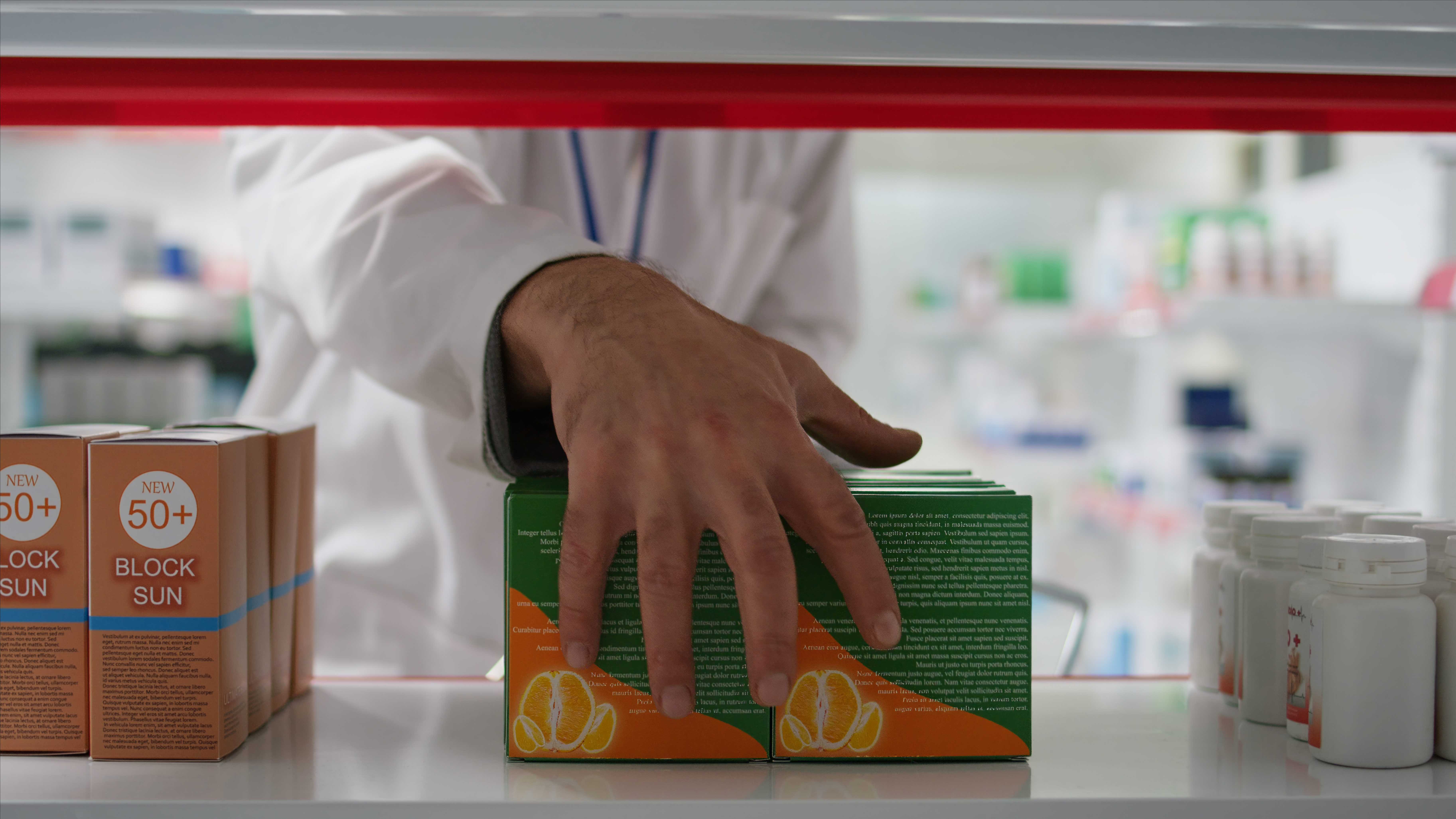
Packaging & E/L Testing
Why: Packaging materials can leach chemicals that affect drug safety.
What we test: Extractables/leachables, compatibility, integrity.
Who needs it: All drug manufacturers (especially injectables, biologics).
Benefits of Testing with Prewel Labs
Accredited & Compliant
NABL-certified lab aligned with global pharmacopeias (IP, USP, BP, EP).
Regulatory Confidence
Support for FDA, EMA, CDSCO, and WHO audits.
Digital Dashboard
Real-time results, audit-ready reports, and predictive foresight.
Patient-Centric
Detect issues before they reach patients.
Faster Approvals
Reduce delays in filings and market launch.

Who We Serve
Pharma Manufacturers
Formulations, injectables, biologics, APIs
Biotech Companies
Drug development and scale-up testing
Contract Manufacturers
(CMOs/CDMOs) – Batch release testing
Exporters
Compliance for US, EU, and global markets
Pharma Testing at a Glance
| Test Type | Why It Matters | Key Parameters | Who Needs It |
|---|---|---|---|
| Raw Material Testing | Prevents poor-quality APIs/excipients from entering production | Identity, purity, potency, microbial limits | API & formulation companies |
| Finished Product Testing | Ensures safety & efficacy of medicines | Assay, dissolution, sterility, microbial limits | Formulation manufacturers |
| Stability Testing | Establishes shelf-life & storage | Accelerated, real-time stability, degradation | All pharma companies |
| Microbial & Sterility Testing | Prevents contamination & patient harm | Sterility, endotoxins, bioburden | Sterile dosage manufacturers |
| Utilities & Process Water | Prevents waterborne contamination | Purified water, WFI, clean steam, gases | All pharma plants |
| Packaging & E/L | Ensures packaging safety & compatibility | Extractables, leachables, integrity | Drug & biologic manufacturers |
